Carnot Cycle: Difference between revisions
From wikiluntti
| Line 8: | Line 8: | ||
== Ideal Gas == | == Ideal Gas == | ||
[[File:CarnotCycle pvDiagram simple.png|thumb]] | |||
<math> pV = nRT</math>, or more genrally polytropic process: <math>pV^\gamma = C</math>, where is different processes depending on the value of the ''n'': | <math> pV = nRT</math>, or more genrally polytropic process: <math>pV^\gamma = C</math>, where is different processes depending on the value of the ''n'': | ||
Revision as of 17:37, 15 August 2024
Introduction
- Isothermal expansion: Heat is transferred from the hot reservoir to the gas.
- Isentropic (reversible adiabatic) expansion: without transfer of heat to or from a system, so that Q = 0, is called adiabatic, and such a system is said to be adiabatically isolated. Eg. the compression of a gas within a cylinder of an engine is assumed to be rapid that little of the system's energy is transferred out as heat to the surroundings.
- Isothermal compression
- Isentropic compression
Ideal Gas
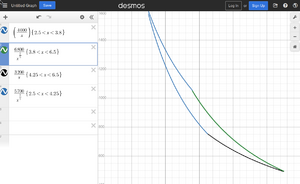
, or more genrally polytropic process: , where is different processes depending on the value of the n:
- n = 0: isobaric
- n = ∞: isochoric
- n = 1: isothermal
- n = γ: isentropic
Adiabatic index Failed to parse (Conversion error. Server ("https://wikimedia.org/api/rest_") reported: "Cannot get mml. Server problem."): {\displaystyle \gamma =c_{p}/c_{v}} is for the air 7/5. For the ideal gas we have and .
- (n=1) Isothermal compression: T is constant, thus we have Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p =C/V } .
- (n=γ) Isentropic Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle p=C/V^\gamma}



